There is a microcap biopharma company that has been building momentum over the last several months, and is now approaching what I believe could be an impactful period of news flow for the company. This is a company that made a transformative acquisition in early 2024 by buying the entire portfolio of discovery, preclinical and clinical development stage assets of former Canadian biotechnology company, IMV Inc., Immunovaccine Technologies Inc., and IMV USA. BioVaxys Technology Corp (CSE:BIOV, OTC: BVAXF) paid US$750,000 and issued five million shares to consummate the acquisition of the IMV portfolio. To put this purchase price into perspective, at one time IMV had a $300 million market cap as a public company, and over its lifetime as a public company IMV had total R&D expenditures of more than $200 million.
Since the IMV acquisition, BioVaxys has been putting the pieces in place to advance its crown jewel immune educating platform technology, DPX™. BioVaxys has been busy during the last month, and this could only be the beginning of a more intense period of impactful news flow for the company.
In early October, BIOV announced that it engaged Rajkannan Rajagopalan, PhD, as Advisor for development and production of BIOV’s DPX™ formulations. Dr. Rajagopalan has a PhD in Pharmaceutical Chemistry/Physical Chemistry, with over 20 years of experience in nanoparticles formulation development for biomolecules (peptides, proteins, nucleic acids, VLPs, mAbs) delivery to treat cancer, infectious diseases and autoimmune disorders. His name is on many of the IMV patents where he developed DPX-based vaccines to treat breast, ovarian, bladder and hard to reach cancers as Senior Director of Formulation Development for IMV.
The addition of Dr. Rajagopalan to the BIOV team is an important step in advancing the DPX platform to the next stages across a broad spectrum of indications including:
- Maveropepimut-S (MVP-S), based on the DPX™ platform, and is in Phase II clinical development for advanced Relapsed-Refractory Diffuse Large B Cell Lymphoma (DLBCL) and platinum resistant Ovarian Cancer
- DPX™+SurMAGE, a dual-targeted immunotherapy combining antigenic peptides for both the survivin and MAGE-A9 cancer proteins to elicit immune responses to these two distinct cancer antigens simultaneously,
- DPX™-RSV for Respiratory Syncytial Virus

Dr. Rajagopalan will be instrumental in supporting BIOV in the development of DPX formulations for the company’s peanut allergy vaccine program with McMaster University, planned preclinical studies of DPX/mRNA vaccines, as well as continued Phase 1 studies in oncology and infectious disease and expansion into new opportunities with DPX. Dr. Rajagopalan will also help BioVaxys establish its own non-GLP clinical supply facility for production of DPX formulations.
DPX Immune Activating Properties Confirmed In Study
On October 31st, BIOV highlighted a study conducted in collaboration with Dalhousie University of Halifax, Nova Scotia in which DPX was compared to aqueous and emulsion-based formulations to evaluate the dynamics of immune cell recruitment to the site of injection, antigen consumption, and trafficking by immune cells. The study demonstrated that DPX is a novel immune-educating delivery system that is more effective at activating immune responses than conventional water-based and emulsion antigen delivery methods. DPX recruits antigen-presenting cells (APCs) over time, providing a sustained immune activation critical for cancer vaccines. Unlike standard methods, DPX retains antigens at the injection site, promoting targeted immune responses without leaking antigens into surrounding tissues. A significant difference between DPX and oil emulsion was that antigen presentation driven by the DPX platform resulted in activation of more critical markers of T cell subsets than emulsion.

An additional and highly significant finding is that recruitment and activation of these T cell subsets by DPX was evident regardless of whether an antigen was contained as cargo in DPX, indicating that DPX on its own has immune system activating properties.
This means that DPX not only outperforms current antigen delivery solutions but also demonstrates inherent immune-activating properties, opening up significant revenue potential for BIOV’s DPX platform across multiple market segments.

Government Validation
To further confirm that BIOV is generating significant momentum, this morning the company announced that BioVaxys has been invited to participate in the US Government Biomedical Advanced Research and Development Authority (BARDA) Rapid Response Partnership Vehicle (RRPV) Vaccine Development Consortium. This means that the US government hand-selected BioVaxys to be a part of this exclusive program due to the promise of the DPX platform.
The Rapid Response Partnership Vehicle (RRPV) is a multi-purpose acquisition framework designed to streamline the full lifecycle of medical countermeasure development—from early research through to commercialization and manufacturing infrastructure. Created to respond swiftly to pandemics and biothreats, the RRPV supports innovation and partnerships for developing vaccines, therapeutics, and related technologies to bolster national health security.
Under BARDA’s guidance, consortium members can propose projects, and selected proposals will receive RRPV-backed agreements. The RRPV complements BARDA's existing tools, such as the Broad Agency Announcement, by providing additional rapid pathways for partnering on projects like improved flu vaccines and fast-response vaccine platforms.
BioVaxys President and Chief Operating Officer Kenneth Kovan applauded BIOV’s inclusion in the RRPV and noted the new possibilities it opens up for the company in terms of collaboration with some of the world’s largest pharma companies:
“We are pleased to join the RRPV consortium with organizations including Astra-Zeneca, Battelle, Deutsches Zentrum für Infektionsforschung e.V. (The German Center for Infection Research), Genentech, Ginkgo Bioworks, Inc., Novavax and Leidos. We believe DPX can play a major role in the development of BARDA’s priority vaccine programs, and having the ability to collaborate with such world-renowned consortium members will no doubt be a significant benefit for the Company.”
BioVaxys is planning to present additional data in early December on the unique characteristics of DPX. The company is building significant momentum and the company’s recent news releases have only scratched the surface of what is possible for the DPX™ delivery platform.
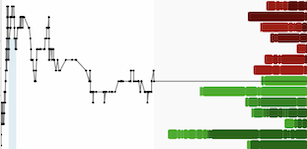
The Chart
BioVaxys Technology (Daily)

BIOV shares are exhibiting signs of accumulation with several high volume green days in recent weeks. The chart shows strong support at $.05, with a breakout above $.08 targeting the next resistance level at the $.10 round number psychological level - a decisive breakout above $.10 would open up blue-sky potential to the next upside resistance level near $.18.
Disclosure: Author owns BioVaxys Technology shares at the time of publishing and may choose to buy or sell at any time without notice. BioVaxys Technology Corp. is a sponsor of Goldfinger Capital.
Disclaimer
The article is for informational purposes only and is neither a solicitation for the purchase of securities nor an offer of securities. Readers are expressly cautioned to seek the advice of a registered investment advisor and other professional advisors, as applicable, regarding the appropriateness of investing in any securities or any investment strategies, including those discussed above. The stocks discussed in this article are high-risk venture stocks and not suitable for most investors. Consult Company SEDAR profiles for important risk disclosures. This interview contains certain forward-looking information and forward-looking statements within the meaning of applicable securities legislation (collectively “forward-looking statements”). Certain information contained herein constitutes “forward-looking information” under Canadian securities legislation. Generally, forward-looking information can be identified by the use of forward-looking terminology such as “expects”, “believes”, “aims to”, “plans to” or “intends to” or variations of such words and phrases or statements that certain actions, events or results “will” occur. Forward-looking statements are based on the opinions and estimates of management as of the date such statements are made and they are subject to known and unknown risks, uncertainties and other factors that may cause the actual results, level of activity, performance or achievements of the Company to be materially different from those expressed by such forward-looking statements or forward-looking information, standard transaction risks; impact of the transaction on the parties; and risks relating to financings; regulatory approvals; foreign country operations and volatile share prices. Although management of the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements or forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Actual results may differ materially from those currently anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements and forward looking information.